Richard W. Fitch - Research
Structural Biology of Nicotinic Receptors
The native ligand for these receptors is acetylcholine (below). However, a number of natural and synthetic ligands have been discovered and/or developed for this receptor, as it is important in analgesia, cognition, addiction, and a variety of other normal and pathological processes. Nicotinic receptors have been implicated in disease states ranging from Alzheimer’s dementia and Schizophrenia to Tourette’s syndrome, Parkinson’s disease, and certain epilepsies, such as autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). These receptors are thus important targets for the development of therapeutics.
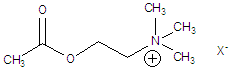
Aside from gross organization of the subunits, little is known about the fine structure of the receptor. Most of what is known comes from the electron diffraction work of Unwin and his co-workers at 9 Å and later at 4.5 Å on the acetylcholine receptor found in the electric organ of Torpedo, an electric fish. However, no atomic scale structure exists, save for that modeled after the acetylcholine binding protein found in a marine flatworm. From Unwin's work, we do have some general parameters of size and shape as shown below.
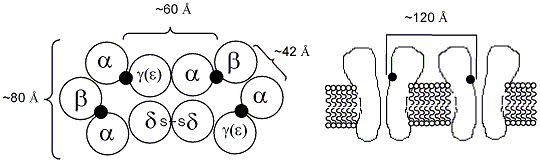
While this information is of use, a detailed analysis of the binding site and gating mechanism would be invaluable. More recently, the x-ray structure of an acetylcholine binding protein from a snail has become available. This protein displays a high homology to the a7 nicotinic receptor and has been used to model the binding sites for a variety of receptor subtypes by virtual mutation. In 2004, this model was combined with the Unwin data to produce a model of a complete nicotinic receptor.
Homologated ligands for nAChRs
Our laboratory is interested in
natural and synthetic
compounds that have activity at nAChRs. A selection of such
compounds is shown below.
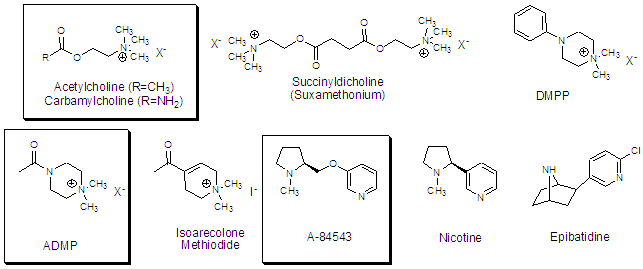
Many of these compounds are active and we are interested in them from two fronts. First, we would like to know the precise structural parameters that make up the pharmacophore, or active structure of the molecule. We are currently studying three ligand series to determine the length of substitution that is tolerated on the molecule while retaining affinity (above, boxed). Each of these series has been shown to support significant substitution on parts of the molecule. For example choline retains affinity when extended at the acyl methyl as evidenced by the biological activity of succinyldicholine to its right, which can be envisioned as a dimer of acetylcholine. Likewise, ADMP, which is an analog of acetylcholine should tolerate substitution by the same argument. The nicotine analog A-84543, developed by Abbott Laboratories, has been shown to allow substitution in the 5 position, as has nicotine and epibatidine, above. We wish to use the A-84543 scaffold simply because of its ease of synthesis as compared to the two natural products.
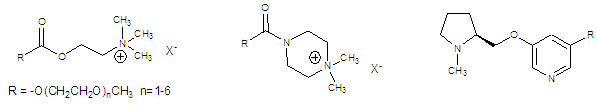
 We have tagged these molecules with polyethylene glycol chains
ranging from one to six units in order to assess the effect on binding.
Initial results suggest this substitution is tolerated and we have less
than ten-fold reduction in affinity (BMC
2008). Since the molecules tolerate
this substitution without losing affinity, then we can safely assume the
tether (up to 20 atoms long, will reach outside the binding pocket into
the channel lumen of the receptor. Thus we can reasonable argue
the orientation of the molecule in the binding site is not substantially
altered from that of the unsubstituted compound. Secondly, the use
of polyethylene glycol linkers is generally biocompatible and make the
molecules quite water soluble relative to ordinary hydrocarbon linkers.
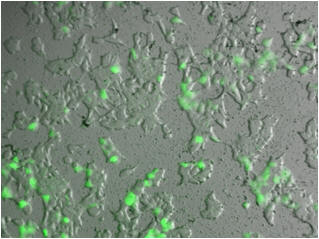
We have since prepared a series of fluorescently labeled versions of
these molecules for visualizing cells expressing nicotinic receptors (ACS
2010). These have demonstrated promise and work is currently
in progress to refine these derivatives as useful biological probes.
We have tagged these molecules with polyethylene glycol chains
ranging from one to six units in order to assess the effect on binding.
Initial results suggest this substitution is tolerated and we have less
than ten-fold reduction in affinity (BMC
2008). Since the molecules tolerate
this substitution without losing affinity, then we can safely assume the
tether (up to 20 atoms long, will reach outside the binding pocket into
the channel lumen of the receptor. Thus we can reasonable argue
the orientation of the molecule in the binding site is not substantially
altered from that of the unsubstituted compound. Secondly, the use
of polyethylene glycol linkers is generally biocompatible and make the
molecules quite water soluble relative to ordinary hydrocarbon linkers.
We have since prepared a series of fluorescently labeled versions of
these molecules for visualizing cells expressing nicotinic receptors (ACS
2010). These have demonstrated promise and work is currently
in progress to refine these derivatives as useful biological probes.