Richard W. Fitch - Research
Pharmacology of Nicotinic Receptors
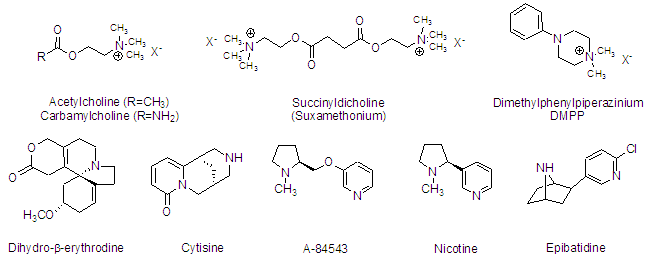
The work in our laboratory centers around one primary biological target, the nicotinic acetylcholine receptor (nAChR). We are primarily interested in the discovery and development of molecules that act at these receptors in order to better understand their function and potentially as therapeutics. There are a variety of compounds that interact with these receptors. A few are shown below.
The biological activity of compounds is intimately linked to structure. If a molecule has the proper functional groups in the proper locations and has the proper overall shape, then it will bind to a site on a protein (enzyme or receptor) and elicit a biological response either by activation of some function the protein or inhibition of its normal activity. Subtle changes in the structure of a molecule can often lead to dramatic changes in its activity. For example, if we were to take epibatidine (left) and look at it, there are a number of possible structural substitutions as shown below.
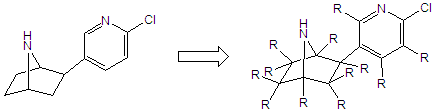
Many of these substitutions will lead to diminished activity, but some will enhance the activity. From a synthetic standpoint, we would find some of these substitutions quite difficult, while others are more facile. However, from a pharmacological standpoint, Nearly all of these are useful in that they tell us the steric parameters (shape) of the binding pocket. Thus for epibatidine and some related ligands, we are trying to substitute the compounds in several places to get a better idea of the steric requirements for binding.
We are interested in the discovery and development of selective ligands for nAChR and especially the molecular requirements for agonism at this receptor. This is a nontrivial matter, as designing molecules to bind to a receptor is a matter of identifying the pharmacophore, or mininmal structure for activity. For antagonists, one set of parameters is required for binding to the receptor in the correct location. Primarily this is a complementary shape argument and has been elegantly addressed from examples using DNA binding and the inhibition of HIV reverse transcriptase by buckminsterfullerene. They bind simply because they fit.
The design of agonists take this one step further, in that they must activate the receptor, inducing the conformational changes required to cause the pore to open. If one considers Fisher's "lock and key" principle, the structural elements for binding are like the slots on your key that allow it to fit into a lock. An agonist must also have the right arrangement of teeth on the key to raise the tumbler pins to allow the lock to turn, which is a much more delicate matter. In chemical terms, it must have the proper spatial arrangement of attractive or groups to allow a movement of residues in the binding site that will translate to the gate in the pore of the receptor.
Nonetheless, efficacy is an important matter when designing compounds that are meant to only partially activate a receptor, as in smoking cessation. Many of the compounds on the market for this are antagonists, that block the action of nicotine, but do nothing to reduce the cravings for it. A partial agonist has the capacity to potentially alleviate this craving while still blocking reinforcement by nicotine or the drug. This is the principle behind the antismoking drug varenicline, (Chantix ® , Pfizer).
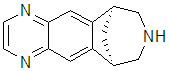
We are trying to evaluate this issue by examing the molecular determinants of efficacy. Efficacy is the ability of a ligand to activate a receptor, the quantitative assessment of agonism. This is a nontrivial matter, as efficacy and potency can be closely linked. Changes in structure generally affect both. We are using a combination of binding and functional assays to sort this issue out. Binding gives us affinity data only, while functional data gives us a mathematically related combination of affinity and efficacy. We hope to tease out these parameters, so that one can design ligands of desired potency and efficacy.