Richard W. Fitch - Research
Natural Product Synthesis
Alkaloid Natural Products - "Izidines"
Our laboratory is interested in the synthesis of
alkaloidal natural products, especially those containing bicyclic and
polycyclic cores containing a bridgehead nitrogen ("izidines"). A
number of natural products possess this skeleton, a few of which are
shown below.
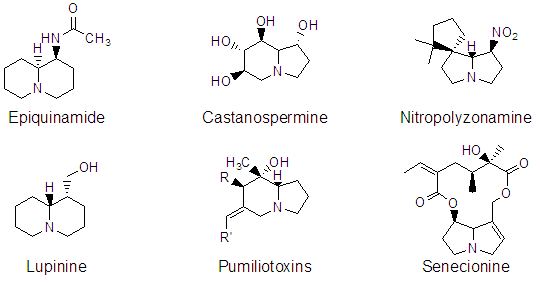
The azabicyclic cores of these alkaloids may be constructed in a number of ways, however, we are interested in a general scheme for synthesis based on tandem N-alkylation-aldol reactions of cyclic imines to accomplish this synthesis as shown below for some quinolizidines.
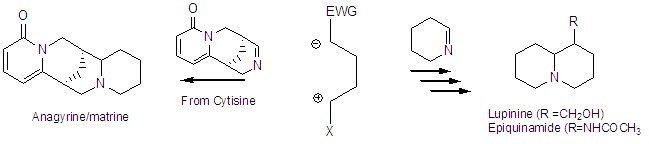
One can envision the cyclization of imines to produce a variety of structures by variation of ring size of the imine and chain length of the homologation synthon. A synthesis of epiquinamide has been completed (JNP 2009) and we are working toward several of the lupine alkaloids at this time. This work has been funded by startup funds and Sigma Xi.
Phantasmidine
Phantasmidine is a condensed tetracyclic alkaloid isolated from an Ecuadoran poison frog (JNP 2010). This is the same frog which produced epibatidine and epiquinamide (JNP 2003), as well as a number of other known (and as yet unknown) alkaloids. Phantasmidine was isolated in tiny quantities (~12 mg) and was derivatized for its structural characterization. Thus its biology was not fully explored at the time.
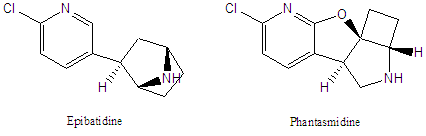
We are currently working on the enantioselective synthesis of this alkaloid and analogs for full evaluation of the biological activity. This novel tetracycle is a rigid analog of epibatidine and may be of use as a tunable nicotinic receptor probe and potential therapeutic. We are exploring ring closing methodology based on rhodium catalyzed transannular C-H insertion for access to this unusual tetracyclic system. This work was initiated with funds the ISU Center for Public Service and Community Engagement and is currently funded by an NSF RUI grant (CHE1012629).
Dioicine
Dioicine is a prenylated purine natural product isolated from the Kentucky coffeetree (Gymnocladus dioicus, Heterocycles 2009). This unusual (and unstable) alkaloids contains a prenyl-derived enamine pendant from the 3-position of the purine nucleus. It is moderately toxic to animals but may explain the use of the seeds of this tree as a coffee substitute as its facile hydrolysis produces a bioisosteric analog of paraxanthine, the primary metabolite of caffeine in man.
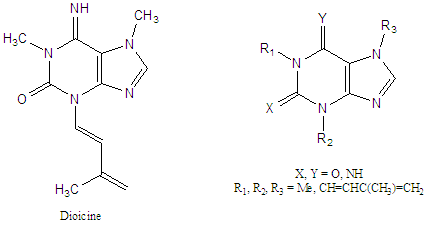
We are investigating the synthesis of this unusual alkaloid from paraxanthine to explore its pharmacology at purinergic receptors as well as that of analogs placing the prenadienyl group at other positions. This work is funded by the Center for Public Service and Community Engagement as well as the University Research Committee (UNR 301).