Richard W. Fitch - Research
Pharmacognosy
Pharmacognosy comes from the greek pharmakos (medicine) and gnosis (knowledge). The science of pharmacognosy deals with the study of drugs or drug substances from natural sources (generally referred to as natural products). This includes the characterization of known natural medicines in their matrix, such as herbal medicines (Gingko biloba, for example). It also deals with the discovery of new drugs from natural sources, including plants, animals, and microbes. Natural products can even be derived from microorganisms that cannot be cultured by looking at the DNA they leave behind. Natural products have been the source or inspiration for nearly half the drugs on the market today. More information can be found at the American Society of Pharmacognosy website.A fair bit of the work in our laboratory deals with the isolation, structure elucidation and synthesis of novel, bioactive natural products. While much is made of the drug potential of natural products, these compounds are far more often used as biological probes to evaluate biochemical mechanisms, selectively activating or inhibiting key receptors and enzymes involved in biological pathways. In this way they are exquisite tools for the pharmacologist. Labeled derivatives can aid in the location and quantitation of their biological targets, thus serving as enabling tools for cell biologists and anatomists, as well as diagnostic tools that can be used in the clinic. We believe this is the true power of natural products.
Bioassay-guided Isolation of Natural Products
Our laboratory is interested in the
isolation and structure elucidation of natural products.
Specifically, we are interested in compounds that have activity at
neuronal nicotinic acetylcholine receptors. These receptors are
ligand-gated ion channels that mediate much of the fast synaptic
transmission in the central nervous system. Some compounds that
have nicotinic activity are shown below. Those in boxes represent natural
compounds (except carbamylcholine) and the others represent compounds
inspired by these natural products.
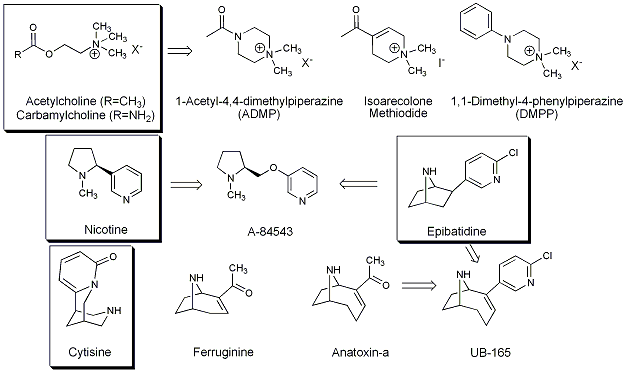
Many of the molecules above are in the class of compounds called alkaloids. Alkaloids are compounds that typically contain a basic, heterocyclic nitrogen, although non-heterocycles, such as the biogenic amines are often lumped into this category. We are primarily interested in alkaloids as this class of compounds contain some of the most interesting biologically active molecules around. For example the pain killer morphine is is an alkaloid isolated from the opium poppy Papaver somniferum. Likewise, the anticancer alkaloid camptothecin is isolated from a chinese tree, Camptotheca accuminata. Alkaloids are relatively easy to isolate from neutrals and acidic compounds by simple acid-base aqueous/organic extractions, provided they are not too polar.
Principally we are interested in alkaloids with activity at nicotinic acetylcholine receptors. Many such alkaloids have been isolated from plant and animal sources. In our laboratory, we use bioassays based on radioligand binding, mobilization of intracellular calcium and alteration of membrane potential (PNAS 2003) to identify compounds which are active at these receptors (JNP 2003).
Alkaloids from Amphibians and Arthropods
 Amphibians
have produced a wealth of alkaloid natural products having a wide
variety of biological activities. Many of these alkaloids have
neurologically relevant mechanisms of action including at nicotinic
receptors (JNP
2003, 2010).
In collaboration with chemists Tom Spande and Martin Garraffo at the
National Institutes of Health, we are examining extracts from
neotropical poison frogs collected by the late John Daly for activities
at nicotinic receptors (such as Epipedobates anthonyi at right). In particular, we are examining minute
extracts, whole and chromatographically separated.
Capillary NMR technology allows
examination of microgram quantites of mixtures and isolated compounds.
We are interested in minor and trace biologically active components of
these extracts to identify novel alkaloids. Many of these extracts are
only available in submilligram amounts and previously could only be
examined by mass spectrometry. We are taking advantage of the new
technology to obtain high resolution NMR data as well to assist in
structure elucidation of as yet unknown alkaloids.
Amphibians
have produced a wealth of alkaloid natural products having a wide
variety of biological activities. Many of these alkaloids have
neurologically relevant mechanisms of action including at nicotinic
receptors (JNP
2003, 2010).
In collaboration with chemists Tom Spande and Martin Garraffo at the
National Institutes of Health, we are examining extracts from
neotropical poison frogs collected by the late John Daly for activities
at nicotinic receptors (such as Epipedobates anthonyi at right). In particular, we are examining minute
extracts, whole and chromatographically separated.
Capillary NMR technology allows
examination of microgram quantites of mixtures and isolated compounds.
We are interested in minor and trace biologically active components of
these extracts to identify novel alkaloids. Many of these extracts are
only available in submilligram amounts and previously could only be
examined by mass spectrometry. We are taking advantage of the new
technology to obtain high resolution NMR data as well to assist in
structure elucidation of as yet unknown alkaloids.
We are also in terested
in the chemical ecology of the frogs from which these alkaloid are
derived. Neotropical poison dart frogs (Dendrobatidae) have
evolved an uptake mechanism for the sequestration of dietary alkaloids,
presumably for chemical defense. We are interested in these frogs
and the arthropods from which they obtain their alkaloids, particularly
as a function of changing habitat. We recently collected specimens
of Dendrobates auratus (right) along with leaf litter arthropods in
Oahu, Hawai'i,with biologist and collaborator
Ralph Saporito
of John Carroll University. These frogs were introduced from
Panama in
the 1930's. These frogs were examined in the 1980's and found to
have an alkaloid profile that differed from their counterparts in thier
native Panama. We are examining whether the alkaloid profile of
the frogs has changed in the 20 years since the last collection.
Such changes have been observed in Argentinian toads (Melanophryniscus
stelzneri) and may be the
result of habitat/climate change.
terested
in the chemical ecology of the frogs from which these alkaloid are
derived. Neotropical poison dart frogs (Dendrobatidae) have
evolved an uptake mechanism for the sequestration of dietary alkaloids,
presumably for chemical defense. We are interested in these frogs
and the arthropods from which they obtain their alkaloids, particularly
as a function of changing habitat. We recently collected specimens
of Dendrobates auratus (right) along with leaf litter arthropods in
Oahu, Hawai'i,with biologist and collaborator
Ralph Saporito
of John Carroll University. These frogs were introduced from
Panama in
the 1930's. These frogs were examined in the 1980's and found to
have an alkaloid profile that differed from their counterparts in thier
native Panama. We are examining whether the alkaloid profile of
the frogs has changed in the 20 years since the last collection.
Such changes have been observed in Argentinian toads (Melanophryniscus
stelzneri) and may be the
result of habitat/climate change.
Alkaloids from Native Indiana Plants
One of the organisms we have been studying of late
is the Kentucky coffeetree, Gymnocladus
dioicus (Fabaceae, K. Koch). This
tree can grow to 60 feet and is sometimes used as an ornamental.
The leaves are doubly compound and very large, sometimes exceeding 1 m
in length (below right) It is dioecious and the female trees
produce seed pods containing 3-6 marble sized seeds. The seeds of this tree were
reputedly roasted as and used to brew a drink used as a coffee
substitute by early settlers. However, this tree is known for
being poisonous to livestock and there is at least one anecdotal report
of a human poisoning by the coffeetree. The toxicity was
reportedly due to the toxic alkaloid cytisine and this is widely cited
among the popular literature and on the
 internet. We have investigated
the alkaloid content of this tree and found no trace of cytisine
in the plant. Thus, the thrust of this project has begun to center
around identifying the compound(s) responsible for the reported toxicity.
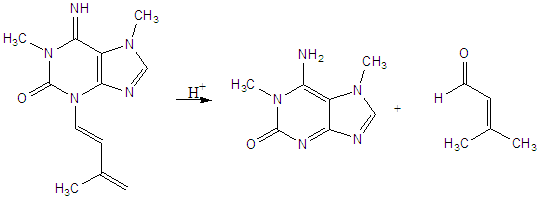
Thus far we have identified a novel prenylated purine alkaloid which we
have named dioicine (ACS
2007,
Heterocycles 2009). This unusual (and unstable) alkaloid
contains a prenyl-derived enamine pendant from the 3-position of the
purine nucleus. It is moderately toxic to animals but may explain
the use of the seeds of this tree as a coffee substitute as its facile
hydrolysis produces a bioisosteric analog of paraxanthine, the primary
metabolite of caffeine in man. We are collaborating with
Kip Panter at the USDA Poisonous Plant Laboratory to investigate
this further.
internet. We have investigated
the alkaloid content of this tree and found no trace of cytisine
in the plant. Thus, the thrust of this project has begun to center
around identifying the compound(s) responsible for the reported toxicity.
Thus far we have identified a novel prenylated purine alkaloid which we
have named dioicine (ACS
2007,
Heterocycles 2009). This unusual (and unstable) alkaloid
contains a prenyl-derived enamine pendant from the 3-position of the
purine nucleus. It is moderately toxic to animals but may explain
the use of the seeds of this tree as a coffee substitute as its facile
hydrolysis produces a bioisosteric analog of paraxanthine, the primary
metabolite of caffeine in man. We are collaborating with
Kip Panter at the USDA Poisonous Plant Laboratory to investigate
this further.