Richard W. Fitch - Research
Synthetic Methodology
Enantioselective sulfur-mediated oxidation of alcohols
The activated sulfoxide oxidation of alcohols (Swern) has been a staple of organic synthesis for many years. It is a mild and highly functional group-tolerant oxidation system.
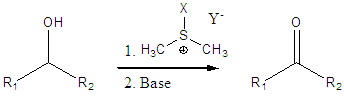
An enantioselective variant of this reaction would be highly desirable. We are investigating the use of chiral sulfoxides to accomplish this transformation, thus affording kinetic resolution of racemic alcohols and/or desymmetrization of meso-diols via selective oxidative removal of one stereocenter.
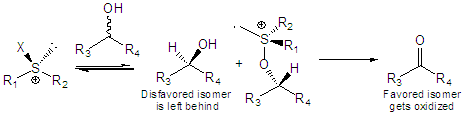
This type of reaction would be highly desirable for a variety of purposes. For example in the synthesis of prostaglandin intermediates via oxidative desymmetrization.
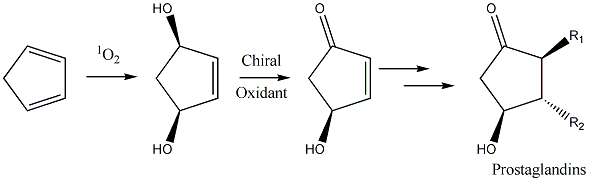
We have examined chiral sulfoxides based on anisole which gave somewhat disappointing results (ACS 2007), likely due to elimination problems. Currently we are looking at chiral sulfoxides derived from cystine and methionine to substitute for DMSO in such reactions(ACS 2008, 2009). In so doing, we have refined a literature procedure for acid-promoted tert-butylation of thiols under modest pressure (< 2 atm) and amenable to parallel synthesis and on mole scale (ACS 2009). This work has been funded by the ISU Office of the Provost and Research Corporation (CCSA6796)