
Chemical Education
Cross-Course Collaboration in the Chemistry Curriculum
It is sometimes perceived that the disciplines of chemistry (analytical,
organic, inorganic, physical and biological) are disjoined and do not
impact one another. It is
unfortunate, yet not entirely rare to hear a chemist denigrate or
disparage divisions outside his/her own, let alone other scientific
disciplines. Such tribalistic
attitudes may be borne of the perception that one group is independent
of another and fostered by a culture of compartmentalization of
disciplinary content (this is not unique to chemistry either).
Undergraduate chemistry is often taught along clear disciplinary
boundaries in self-contained units that don't necessarily emphasize the
interconnectedness of the divisions. This has lessened in
recent years with the emphasis that has been placed on multidisciplinary
research for answering the larger questions in science and technology.
However, interdisciplinary and interdivisional interactions
within chemistry (and all science) need to find their way into the
curriculum. No division or
discipline is entirely independent of any other, whether in chemistry or
any other science.
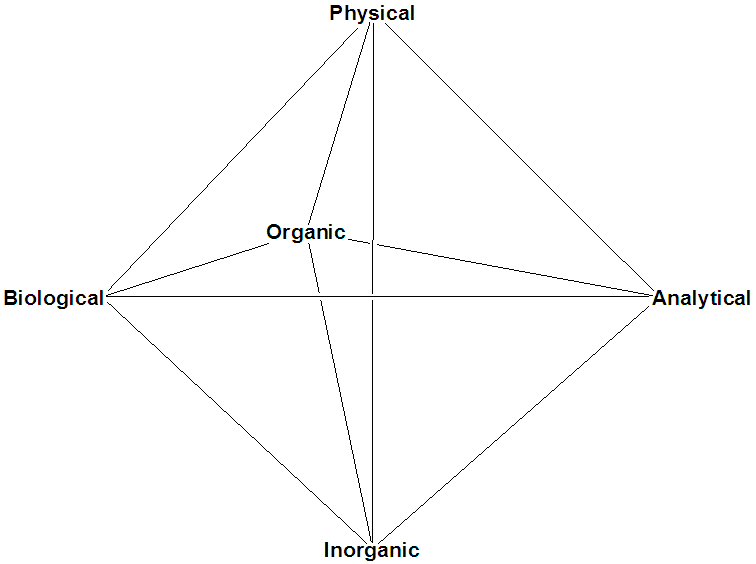
In reality, the relationship between the disciplines is fully
multimodal. Every division uses techniques and tools borrowed from
other divisions in chemistry and from other disciplines altogether (e.g.
physics, biology). For a scientist, getting too hung up on how one
defines himself/herself is not particularly beneficial for the
individual or the practice. In short, virtually no one is a pure
scientist of any kind. Thus, the teaching of science must become more
interdisciplinary, particularly at the undergraduate level, where the
basic concepts are taught and learned. We have embarked on a
project funded by the National Science
Foundation to provide collaborative experiences to chemistry
students in our department. We hope to improve the teaching of
chemistry through fostering a deeper understanding of not only the
content, but the interactions between disciplines. In this way, we
also hope to improve student engagement, a necessary criterion for
learning.
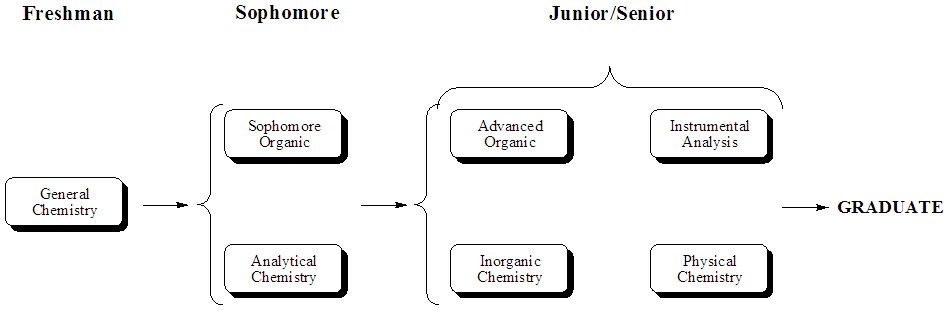
In this project, we look to promote interaction between chemistry
students across divisional lines to accomplish two objectives. This
study focuses on four laboratory courses; sophomore organic, advanced
organic, inorganic and physical chemistry. Each cohort of students
exchanges material or data/interpretation with another group. The
initial focus group is the sophomore organic, as it is the first
disciplinary course taken by the undergraduate chemistry major and lays
the foundations for how one interacts with other disciplines.
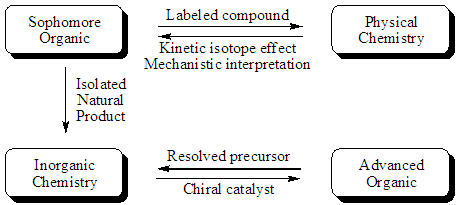
|
The sophomore organic students interact with two other course groups.
First, they provide a labeled compound to the physical chemistry group
who then conduct a kinetics experiment to determine the isotope effect
in a particular reaction and determine if the effect is primary or
secondary based on the magnitude of the effect. These students
then report their results to the organic students. Similar
exchanges occur between the sophomore organic students and the inorganic
students who recieve limonene, a diene obtained from orange peels.
The inorganic students conduct a regioselective reduction of the
molecule using a modified hydrogenation catalyst which they synthesize
and likewise report the results of their experiment to the organic
students. The organic students get a sense that their compound is useful
for something other than sitting in a vial until the professor
disposes of it. They also share a responsibility for the outcome
of the subsequent experiment based on the quality of the material they
provide. The students in the other courses get an appreciation for
where their compounds come from and in completion of the cycle, the
sophomore organic donors ultimately become the recipients of material
from subsequent cohorts and provide results back to them. In this
way, each student progressing through the curriculum gets to see both
sides of the project and hopefully a full appreciation of how the
disciplines work together bringing different expertise to bear on a
chemical problem.
Visualization Tools for Chemistry Learning
Learning many chemistry concepts is a
significantly visual process, particularly in structural contexts.
Organic and other chemists use models to visualize how atoms are
assembled into molecules, including bond lengths and angles.
Often, such models are of the plastic ball-and-stick variety and these
models can be good for teaching concepts such as bond distances and
angles. Rotation of bonds can be used to show conformational
effects. However, such models connected by physical plastic
connectors fail to convey the electrostatic and dynamic effects that
lead to successful bonding.
Magnetic models,
recently introduced by manufacturers such as
Indigo Instruments who market the
well-known
molymod line of model kits may be useful in visualizing such
electrostatic interactions through magnetism as a surrogate.
Magnets are polarized north and south, providing a complementarity
analgous to the positive and negative interactions of atoms based on
charge, whether covalent, ionic or polar.
|